ALK (Anaplastic Lymphoma Kinase)
An important companion diagnostic marker for NSCLC
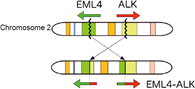
ALK (anaplastic lymphoma kinase) is also known as CD246, is a kinase receptor. ALK came into spot light when it is discovered that its translocation, such as EML4-ALK, creates a driver oncogenic gene in non-small cell lung cancer (NSCLC). A small percentage of NSCLC patients carry EML4-ALK translocation or its variants, which product oncogenic fusion protein.
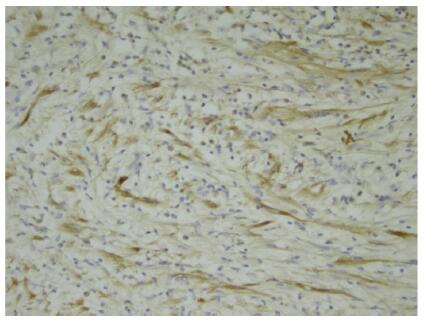
Screening for this fusion gene in NSCLC is important, as "ALK-positive" tumors are highly sensitive to therapy with ALK-targeted inhibitors, such as Crizotinib. ALK translocation is detected by FISH (gold standard), IHC and RT-PCR. For the IHC diagnosis, it is a pivotal to use a highly sensitive antibody as the level of ALK expression is low in NSCLC samples.
- ALK in Scientific Publications
- ALK in Pharma/Biotech
- ALK in Translational Research
- ALK in Clinical Trial
- ALK in News
Recommended ALK products
The use of this Antibody has been cited in the following citations:
Antibody 1A4 with routine immunohistochemistry demonstrates high sensitivity for ALK rearrangement screening of Chinese lung adenocarcinoma patients: A single-center large-scale study, Qiushi Wang, Lianhua Zhao, Xin Yang, Shirong Wei, Ying Zeng, Chengyi Mao, Li Lin,Ping Fu, Liang Lyu, Zengpeng Li, Hualiang Xiao, Lung Cancer Feb 2016 [ALK]
NordiQC Immunohistochemistry Quality Control Run 45 2015 on Lung Anaplastic Lymphoma Kinase (Lu-ALK), Nordi QC Team, NordiQC assessment Run 45 on ALK [ALK]
Inflammatory myofibroblastic tumour of the bladder in children: A review, Collin, M;Charles, A;Barker, A;Khosa, J;Samnakay, N;, J Pediatr Urol Apr 2015 [ALK]
A Novel, Highly Sensitive ALK Antibody 1A4 Facilitates Effective Screening for ALK Rearrangements in Lung Adenocarcinomas by Standard Immunohistochemistry, Gruber K, Kohlhäufl M, Friedel G, Ott G, Kalla C., J Thorac Oncol. 2015 Apr;10(4):713-6 [ALK]
Extraordinary response to crizotinib in a woman with squamous cell lung cancer after two courses of failed chemotherapy, Wang, Q;He, Y;Yang, X;Wang, Y;Xiao, H;, BMC Pulm Med Jun 2014 [ALK]
ALK in Scientific Publications
TKI Sensitivity Patterns of Novel Kinase-domain Mutations Suggest
Therapeutic Opportunities for Patients with Resistant ALK+ Tumors,
Oncotarget, Mar 2016
More...
ALK in Pharma / BioTech
- Novartis (Ceritinib)
- Pfizer (Crizotinib )
- Hoffmann-La Roche (Alectinib)
- Abbott (FISH, FDA Approved in USA)
- Vantana (IHC, Approved in China and EU)
ALK in Translational Research
Non–Small Cell Lung Cancer Cells Acquire Resistance to the ALK Inhibitor Alectinib by Activating Alternative Receptor Tyrosine Kinases, Cancer Research, Mar 2016
ALK in Clinical Trial
- Comparison of qPCR to IHC and FISH for Detection of ALK Fusion Mutations in FFPE Tissue From NSCLC Patients (Enrolling by invitation)
- Retrospective Epidemiology Study Of ALK Rearrangement In Non-Small Cell Lung Cancer Patients In The Middle East & North Africa (Recurring)
ALK in News
March 14 2016, Ceritinib Produces Durable Whole-body Responses in NSCLC
ALK-rearranged NSCLC is sensitive to ALK inhibitors such as crizotinib, but resistance frequently develops, often with metastases to the brain. Ceritinib is a more potent ALK inhibitor than crizotinib in vitro, crosses the blood–brain barrier in vivo, and shows clinical responses in patients with crizotinib-resistant disease.
March 11, 2016, FDA expands use of Xalkori to treat rare form of advanced non-small cell lung cancer
The U.S. Food and Drug Administration today approved Xalkori (crizotinib) to treat people with advanced (metastatic) non-small cell lung cancer (NSCLC) whose tumors have an ROS-1 gene alteration. Xalkori is the first and only FDA approved treatment for patients with ROS-1 positive NSCLC.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China