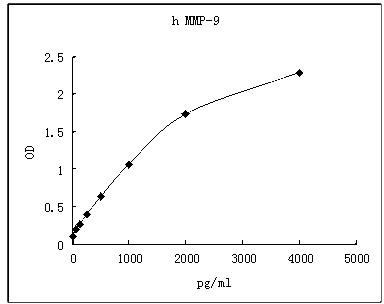
| Background |
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that degrade extracellular matrix proteins . They are secreted as zymogens (Pro-MMPs) that are activated by a variety of proteinases or by reaction with organic mercurials. They are inhibited by specific tissue inhibitors of metalloproteinases (TIMPs) and by 2-macroglobulin . The regulation of MMP activity is important in tissue remodeling, inflammation, tumor growth and metastasis . Human MMP-9 (also known as gelatinase B) is secreted as a 92 kDa zymogen . Cleavage of Pro-MMP-9 at or near residue 87 results in the active enzyme with a mass of approximately 82 kDa . MMP-9 has three fibronectin type II domains, a hemopexin-like domain and a proline-rich type V collagen-homologous domain . Pro-MMP-9 can be activated by MMP-3 or by certain bacterial proteinases . MMP-9 is inhibited by.2-macroglobulin or by TIMP-1 , which binds to Pro-MMP-9 as well as to active MMP-9 . In vitro treatment of Pro-MMP-9 with 4-aminophenylmercuric acid (APMA) produces not only the 82 kDa active enzyme but also a C-terminal truncated form of approximately 65 kDa with the activity comparable to that of the 82 kDa form . Pro-MMP-9 is secreted by monocytes, macrophages, neutrophils, keratinocytes, fibroblasts, osteoclasts, chondrocytes, skeletal muscle satellite cells, endothelial cells, and various tumor cells(1, 7- 21). Pro-MMP-9 expression is upregulated by TGF-.1, IL-1., TGF-., PDGF-AB, TNF-., and IL-1. . Substrates for MMP-9 include denatured collagen type I (gelatin), native collagens type IV, V, VII, X and XI, fibrinogen, vitronectin, IL-1., and entactin, a molecule that bridges laminin and type IV collagen . |