VEGF-E (ov-HB) Orf Virus Protein
Specifications
| Product Data | |
| Species | Orf Virus |
| Expression Host | Insect |
| Predicted MW | 44 kDa |
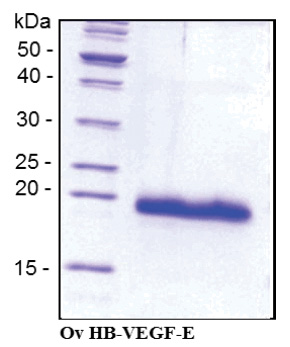
| Purity | >90% pure by SDS-PAGE and visualised by silver stain |
| Presentation | Purified |
| Buffer | Presentation State: Purified State: Lyophilized purified protein Buffer System: 50 mM Acetic Acid, without stabilizer |
| Bioactivity | Biological: Measured by its ability to stimulate 3H-thymidine incorporation in human macrovascular (HUVE) and microvascular (HDME) endothelial cells. The ED50 for this effect is typically 5-20 ng/ml. Range: 1-30 ng/ml. |
| Endotoxin | < 0.1 ng per µg of VEGF-E |
| Reconstitution | The lyophilised HB-VEGF-E is soluble in water and most aqueous buffers. The lyophilised HB-VEGF-E should be reconstituted in PBS or medium containing at least 0.1% Human or Bovine Serum Albumin to a concentration not lower than 50 µg/ml. |
| Preparation | Lyophilized purified protein |
| Protein Description | A DNA sequence encoding the first 116 amino acid residue of Orf virus VEGF-E isolate D1701 (Dehio et al., 1999 EMBO J. 18:363-374; GenBank accession No. AF106020) was fused with a DNA sequence encoding to the C-terminal heparin binding domain of human VEGF165. The chimeric protein was expressed in insect cells using a baculovirus expression system. |
| Storage | Store lyophilized at 2-8°C for 6 months or at -20°C long term. After reconstitution store the antibody undiluted at 2-8°C for one month or (in aliquots) at -20°C long term. Avoid repeated freezing and thawing. |
| Stability | Shelf life: one year from despatch. |
| Reference Data | |
| Summary | Based on sequence similarity to VEGF-A, a gene encoding a VEGF homologue has recently been discovered in the genome of Orf virus (OV) (Lyttle et al., 1994 J. Virol 68:84-92). Different isolates of orf virus show significant amino acid sequence similarity to VEGF-A and described as a viral virulence factor that appear to be derived from captured host genes. All eight cysteine residues of the central cysteine knot motif characteristic of members of the VEGF family are conserved among other residues in the VEGF-E proteins (Dehio et al., 1999 EMBO J. 18:363-374; Wise et al., 1999 Proc. Natl. Acad. Sci USA 96:3071-3076). Alignment of all mammalian VEGF sequences indicated that VEGF-E is distinct from the previously described VEGFs but most closely related to VEGF-A. Like VEGF-A, VEGF-E was found to bind with high affinity to VEGF receptor-2 (KDR) resulting in receptor autophosphorylation, whilst in contrast to VEGF-A, VEGF-E and hb-VEGF-E can not bind to VEGF receptor-1 (Flt-1). Therefore VEGF-E is a potent angiogenic factor selectively binding to VEGF receptor–2/ KDR. Compared to human VEGF165 this virus form has no heparin-binding domain and seems to be a freely secreted protein comparable to VEGF121. In order to compare this form with human VEGF165, an additional heparin-binding domain was engineered at the C-terminus to allow interaction with proteo-aminoglycans and heparan sulfate. These form is also able to interact with neuropillin–1. |
Documents
| FAQs |
| SDS |
Resources
Recombinant Protein Resources |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China