beta B1 Crystallin (CRYBB1) (NM_001887) Human Mass Spec Standard
CAT#: PH307718
CRYBB1 MS Standard C13 and N15-labeled recombinant protein (NP_001878)
Frequently bought together (2)
Other products for "beta B1 Crystallin"
Specifications
| Product Data | |
| Tag | C-Myc/DDK |
| Species | Human |
| Expression Host | HEK293 |
| Expression cDNA Clone or AA Sequence | RC207718 |
| Predicted MW | 28 kDa |
| Protein Sequence |
>RC207718 protein sequence
Red=Cloning site Green=Tags(s) MSQAAKASASATVAVNPGPDTKGKGAPPAGTSPSPGTTLAPTTVPITSAKAAELPPGNYRLVVFELENFQ GRRAEFSGECSNLADRGFDRVRSIIVSAGPWVAFEQSNFRGEMFILEKGEYPRWNTWSSSYRSDRLMSFR PIKMDAQEHKISLFEGANFKGNTIEIQGDDAPSLWVYGFSDRVGSVKVSSGTWVGYQYPGYRGYQYLLEP GDFRHWNEWGAFQPQMQSLRRLRDKQWHLEGSFPVLATEPPK TRTRPLEQKLISEEDLAANDILDYKDDDDKV |
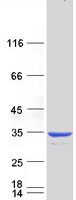
| Purity | > 80% as determined by SDS-PAGE and Coomassie blue staining |
| Concentration | >0.05 µg/µL as determined by microplate BCA method |
| Labeling Method | Labeled with [U- 13C6, 15N4]-L-Arginine and [U- 13C6, 15N2]-L-Lysine |
| Buffer | 25 mM Tris-HCl, 100 mM glycine, pH 7.3 |
| Storage | Store at -80°C. Avoid repeated freeze-thaw cycles. |
| Stability | Stable for 3 months from receipt of products under proper storage and handling conditions. |
| Reference Data | |
| RefSeq | NP_001878 |
| RefSeq Size | 921 |
| RefSeq ORF | 756 |
| Synonyms | CATCN3; CTRCT17 |
| Locus ID | 1414 |
| UniProt ID | P53674 |
| Cytogenetics | 22q12.1 |
| Summary | Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a superfamily. Alpha and beta families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Beta-crystallins, the most heterogeneous, differ by the presence of the C-terminal extension (present in the basic group, none in the acidic group). Beta-crystallins form aggregates of different sizes and are able to self-associate to form dimers or to form heterodimers with other beta-crystallins. This gene, a beta basic group member, undergoes extensive cleavage at its N-terminal extension during lens maturation. It is also a member of a gene cluster with beta-A4, beta-B2, and beta-B3. [provided by RefSeq, Jul 2008] |
Documents
| FAQs |
| SDS |
Resources
Recombinant Protein Resources |
Other Versions
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China