Cd5 Mouse Monoclonal Antibody [Clone ID: CG16]
Other products for "Cd5"
Specifications
| Product Data | |
| Clone Name | CG16 |
| Applications | CT, FC |
| Recommended Dilution | Flow Cytometry Analysis (see Protocols). Cytotoxic Depletion of CD5 Positive Lymphocytes (see Protocols). Functional Analysis (see Protocols). Cytotoxicity Analysis (see Protocols). |
| Reactivities | Mouse |
| Host | Mouse |
| Isotype | IgG2b |
| Clonality | Monoclonal |
| Immunogen | C3H.CE - Ly 1.2 : DS. Donor: C3H spleen. Fusion Partner: Myeloma SP2/0 - Ag 14 (M5). |
| Specificity | This anti-mouse CD5 (Ly 1.2) monoclonal antibody reacts with T cells from mouse strains expressing the Ly 1.2 phenotype. Can be used to identify T helper cells as they have a high density of Ly 1 on their surface. |
| Formulation | PBS, 0.02% NaN3 State: Purified State: Liquid purified IgG |
| Concentration | lot specific |
| Purification | Protein G Chromatography |
| Conjugation | Unconjugated |
| Storage | Store the antibody undiluted at 2-8°C for one month or (in aliquots) at -20°C for longer. Avoid repeated freezing and thawing. |
| Stability | Shelf life: one year from despatch. |
| Gene Name | CD5 antigen |
| Database Link | |
| Background | CD5 is a 55kDa T lymphocyte single chain transmembrane glycoprotein. It is present on all mature T lymphocytes, on most thymocytes and on many T cell leukemias and lymphomas. It reacts with a subpopulation of activated B cells. CD5/Lyt1 antigen is a monomeric type I transmembrane glycoprotein expressed on thymocytes, T lymphocytes, and a subset of B lymphocytes, but not on natural killer (NK) cells. It has been identified as the major ligand of the B cell antigen CD72. The frequency of CD5+ B cells exhibits strain dependent variation, and the phenotypic, anatomical, functional, developmental, and pathological characteristics of the CD5+ B cells suggest that they may represent a distinct lineage, known as B1 cells. Binding of CD5 on the T cell surface can augment alloantigen or mitogen induced lymphocyte proliferation and induces increased cytosolic free calcium, IL2 secretion, and IL2R expression. It has been proposed that CD5 negatively regulates signal transduction mediated by the T cell and B cell receptors. |
| Synonyms | CD5, LEU1 |
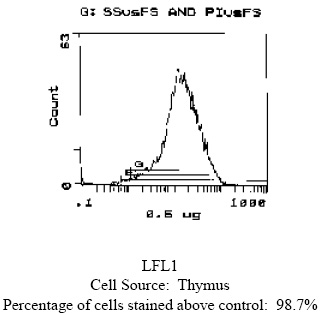
| Note | Protocol: FLOW CYTOMETRY ANALYSIS: Method: 1. Prepare a cell suspension in media A. For cell preparations, deplete the red blood cell population with Lympholyte®-M cell separation medium. 2. Wash 2 times. 3. Resuspend the cells to a concentration of 2x10e7 cells/ml in media A. Add 50µl of this suspension to each tube (each tube will then contain 1x10e6 cells, representing 1 test). 4. To each tube, add 0.1-0.5µg* of this Ab. 5. Vortex the tubes to ensure thorough mixing of antibody and cells. 6. Incubate the tubes for 30 minutes at 4°C. 7. Wash 2 times at 4°C. 8. Add 100 µl of secondary antibody (FITC Goat anti-mouse IgG (H+L)) at a 1:500 dilution. 9. Incubate the tubes at 4°C for 30-60 minutes. (It is recommended that the tubes are protected from light since most fluorochromes are light sensitive). 10. Wash 2 times at 4°C in media B. 11. Resuspend the cell pellet in 50 µl ice cold media B. 12. Transfer to suitable tubes for flow cytometric analysis containing 15 µl of propidium iodide at 0.5 mg/ml in PBS. This stains dead cells by intercalating in DNA. Media: A. Phosphate buffered saline (pH 7.2) + 5% normal serum of host species + sodium azide (100 µl of 2M sodium azide in 100 mls). B. Phosphate buffered saline (pH 7.2) + 0.5% Bovine serum albumin + sodium azide (100 µl of 2M sodium azide in 100 mls). Results - Tissue Distribution: Mouse Strain: BALB/c Cell Concentration: 1x10e6 cells per tests Antibody Concentration Used: 0.5 µg/10e6 cells Isotypic Control: Mouse IgG2b CYTOTOXICITY ANALYSIS: Method: 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Mediuma or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte®-Mb density cell separation medium. After washing, adjust the cell concentration to 1x10e6 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1:500 and mix. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox®-M Rabbit Complementc diluted 1:12 in Cytotoxicity Medium. 6. Incubate for 60 minutes at 37°C. 7. Place on ice. 8. Add Trypan Blue, 10% by volume of 1% Trypan Blue (w/v) added 3-5 minutes before scoring works well. Score live versus dead cells in a hemacytometer. Notes: a. Cytotoxicity Medium is RPMI-1640 with 25 mM Hepes buffer and 0.3% bovine serum albumin (BSA). BSA is substituted for the conventionally used fetal calf serum (FCS) because we have found that many batches of FCS contain complement dependent cytotoxins to mouse lymphocytes, thus increasing the background killing in the presence of complement. We recommend that cells not be exposed to FCS prior to or during exposure to antibody and complement. Some batches of BSA also contain complement dependent cytotoxins, resulting in the same problem. We screen for batches of BSA giving low background in the presence of complement and use the selected BSA for preparing Cytotoxicity Medium. b. Lympholyte®-M cell separation medium is density separation medium designed specifically for the isolation of viable mouse lymphocytes. This separation medium provides a high and non-selective recovery of viable mouse lymphocytes, removing red cells and dead cells. The density of this medium is 1.087 - 1.088. Isolation of mouse lymphocytes on cell separation medium of density 1.077 will result in high and selective loss of lymphocytes and should be avoided. c. Rabbit serum provides the most potent source of complement for use with antibodies to mouse cell surface antigens. However, rabbit serum itself is very toxic to mouse lymphocytes. Low-Tox®-M Rabbit Complement is absorbed to remove toxicity to mouse lymphocytes, while maintaining its high complement activity. When used in conjunction with Cytotoxicity Medium, this reagent provides a highly potent source of complement with minimal background toxicity. Results - Antibody Titration Cell Source: Thymocytes Donor: BALB/c Cell Concentration: 1.1x10e6 cells/ml Complement: Low-Tox®-M Rabbit Complement Complement Concentration: 1:12 Procedure: Two-stage cytotoxicity Results - Tissue Distribution Antibody Concentration: 1:500 Strain: BALB/c Results - Strain Distribution Antibody Concentration Used: 1: 500 Strains Tested: BALB/c, AKR/J, ATH, CBA/J, C3H/He Positive: BALB/c, AKR/J, ATH Negative: CBA/J, C3H/He FUNCTIONAL ANALYSIS: Method: Cells were treated as described in Cytotoxic Depletion of CD5 (Ly 1.2) Positive Lymphocytes. Treated cells and controls were tested for: a) the ability to generate plaque-forming cells (PFC) using a modified Jerne haemolytic plaque assay b) the ability to generate cytotoxic T effector cells using a cytotoxic lymphocyte reaction (CTL) assay. Cells were treated both before and after sensitization in the CTL assay. In vitro immunizations were used in all experiments. Results: Cell Source: Splenocytes Donors: C57BL/6 and C3H/He Cell Concentration: 1x10e7 cells/ml Antibody Concentration used: 1:10 Complement: Low-Tox®-M Rabbit Complement Complement Concentration: 1:10 Treatment of C57BL/6 splenocytes with anti-CD5 (Ly 1.2) plus complement was found to reduce the number of plaque-forming cells and inhibit cytotoxic T cell generation. Cytotoxic T effector cell function was not affected (cells treated after sensitization). No effect was observed when C3H/He cells were used. These results are consistent with the depletion of T helper cells of the Ly 1.2 phenotype. CYTOTOXIC DEPLETION OF CD5 (Ly 1.2) POSITIVE LYMPHOCYTES: Method: 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte®-M density cell separation medium. After washing, adjust the cell concentration to 1x10e7 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1:500 and mix. Alternatively, pellet the cells and resuspend in antibody diluted 1:500 in Cytotoxicity Medium. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox®-M Rabbit Complement, diluted to the appropriate concentration in Cytotoxicity Medium. (Recommended concentration included with each batch of Low-Tox®-M Rabbit Complement.) 6. Incubate for 60 minutes at 37° C. 7. Monitor for percent cytotoxicity at this stage, before further processing. For this purpose remove a small sample from each tube, dilute 1:10 with medium, and add 1/10 volume of 1% Trypan Blue. After 3-5 minutes, score live versus dead cells in a hemacytometer. 8. For functional studies, remove the dead cells from the treated groups before further processing, particularly if the treated cells are to be cultured. This can be done by layering the cell suspension with separation medium and centrifuging at room temperature as per the instructions provided. Live cells will form a layer at the interface, while the dead cells pellet. The interface can then be collected and washed in Cytotoxicity Medium before being resuspended in the appropriate medium for further processing. Alternatively, the cells can be washed and resuspended in the appropriate medium for further processing immediately after Step #6, provided that the dead cells will not interfere with subsequent assays. |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China