Flow Cytometry solutions for evaluating CAR T-cell expression
What are CAR T-cells?
CAR T-cells, also known as chimeric antigen receptor T cells, are a type of immunotherapy used in the treatment of certain types of cancer. They play a crucial role in the body's immune system by recognizing and eliminating abnormal cells, including cancer cells.
CAR T-cell therapy genetically alters a patient's T cells with a chimeric antigen receptor (CAR), a synthetic receptor, fusing antibody precision with the T cell's immune-triggering capacity. This CAR targets and attaches to cancer cell surface proteins, activating the T-cell for a robust immune assault and eventual cancer cell eradication.
CAR T-cell immunotherapy created new hope in the fight against cancer. As of September 2023, there are six CAR T-cell therapies approved by the FDA. While these therapies target B-cell cancers and multiple myeloma, numerous ongoing clinical trials are investigating the efficacy of CAR T–cell therapies against solid tumors1.
How are CAR T-cell therapies developed?
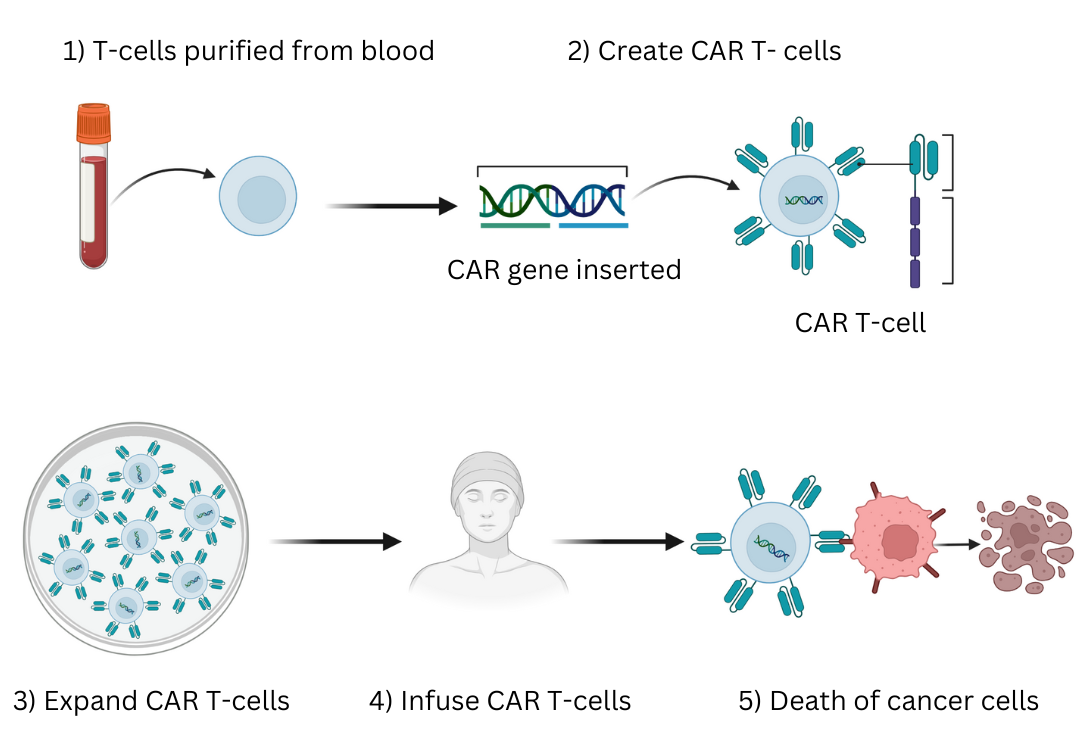
Generating CAR T-cells involves several steps. T cells are first isolated from a donor, activated, and transduced to insert the CAR gene. The cells are then expanded, and a number of tests are done to confirm characteristics of the transduced cells, including whether the T-cells are expressing the CAR.
How is CAR T-cell expression evaluated?
Evaluation of CAR protein expression is critical, since without proper expression, the CAR T-cell cannot function as intended, to eliminate the targeted cancer cell. CAR protein expression is confirmed by a number of methods, including flow cytometry, using CAR target antigens conjugated to a fluorescent tag such as Phycoerythrin (PE) or Allophycocyanin (APC). This direct detection approach is a preferred choice because of low background and high specificity for the CAR. However, indirect methods using biotinylated or unconjugated antigens in combination with fluorescently labeled antibodies provide additional options for detection.
OriGene solutions for accelerating CAR T- cell expression verification
Choose your Flow Cytometry detection option:1) Use recombinant proteins (CAR antigens) conjugated to a fluorophore
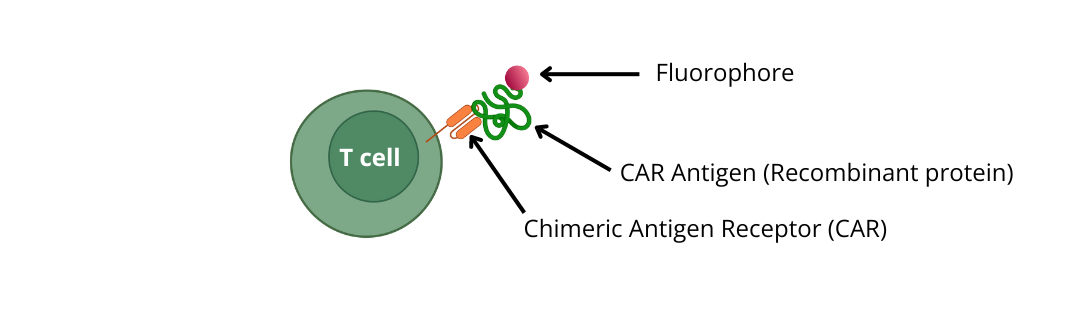
Choose CAR Antigen – PE or APC
2) Use biotinylated recombinant proteins (CAR antigens) and detect with Streptavidin conjugated to a fluorophore
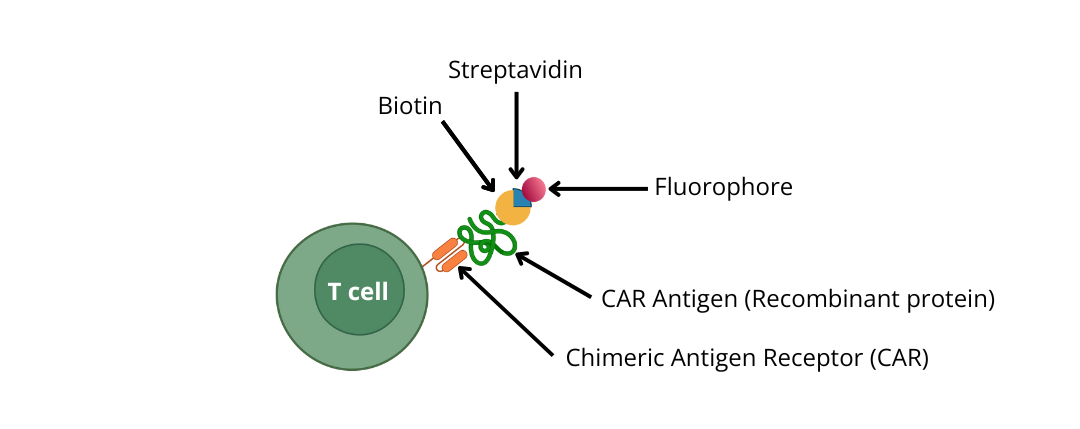
Choose biotinylated CAR Antigen and detection tools
3) Use epitope -tagged recombinant proteins (CAR antigens) and detect with an anti-tag antibody conjugated to a fluorophore
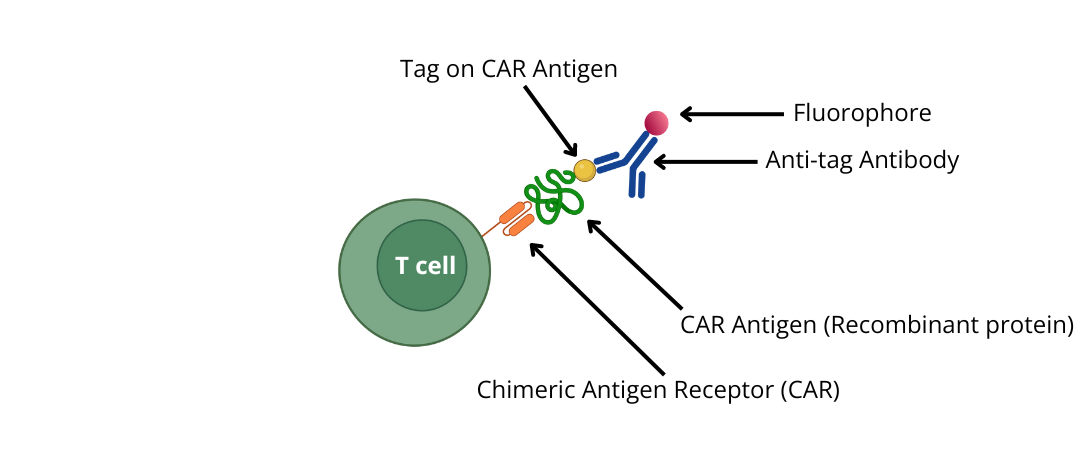
Choose tagged CAR Antigen and detection tools
Search for additional CAR Targets and corresponding antibodies.
Detection of PE conjugated BCMA recombinant protein by flow cytometry.
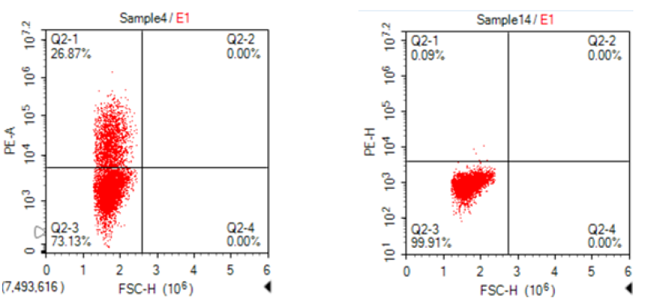
A: BCMA CAR – transfected CHO cells B: Mock-transfected CHO cells
Transfected CHO cells were stained with PE conjugated BCMA-Fc protein (TP721285) at 4.0 µg/mL for 1 hr at RT.
Learn about our custom solutions to accelerate your CAR T research.
Custom SolutionsReferences
1. Mitra A, Barua A, Huang L, Ganguly S, Feng Q, He B. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. 2023 May 15;14:1188049. doi: 10.3389/fimmu.2023.1188049. PMID: 37256141; PMCID: PMC10225594.
Diagrams generated with Biorender






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China