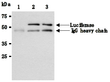
| Note |
This product was originally produced by MBL International.
Protocol: SDS-PAGE & Western Blotting
1) Wash the cells 3 times with PBS and suspend with 10 volumes of cold Lysis buffer (50 mM Tris-HCl, pH 7.2, 250 mM NaCl, 0.1% NP-40, 2 mM EDTA, 10% glycerol) containing appropriate protease inhibitors. Incubate it at 4 oC with rotating for 30 minutes, then sonicate briefly (up to 10 seconds).
2) Centrifuge the tube at 12,000 x g for 10 minutes at 4 oC and transfer the supernatant to another tube. Measure the protein concentration of the supernatant and add the Lysis buffer to make an 8 mg/mL solution.
3) Mix the sample with an equal volume of Laemmli’s sample buffer.
4) Boil the samples for 2 minutes and centrifuge. Load 10 µL of the sample per lane in a 1 mm thick SDS-polyacrylamide gel for electrophoresis.
5) Blot the protein to a polyvinylidene difluoride (PVDF) membrane at 1 mA/cm2 for 1 hour in a semi-dry transfer system (Transfer Buffer: 25 mM Tris, 190 mM glycine, 20% MeOH). See the manufacture's manual for specific transfer procedure.
6) To reduce nonspecific binding, soak the membrane in 10% skimmed milk (in PBS, pH 7.2) for 1 hour at room temperature, or overnight at 4 oC.
7) Incubate the membrane with primary antibody diluted with PBS, pH7.2 containing 1% skimmed milk as suggested in the APPLICATIONS for 1 hour at room temperature. (The optimal antibody concentration will depend on the experimental conditions.)
8) Wash the membrane with PBS (5 minutes x 6 times).
9) Incubate the membrane with the 1:10,000 HRP-conjugated anti-Mouse IgG diluted with 1% skimmed milk (in PBS, pH 7.2) for 1 hour at room temperature.
10) Wash the membrane with PBS (5 minutes x 6 times).
11) Wipe excess buffer from the membrane, then incubate it with appropriate chemiluminescence reagents for 1 minute. Remove extra reagent from the membrane by dabbing with a paper towel, and seal it in plastic wrap.
12) Expose to X-ray film in a dark room for 10 minutes. Develop the film as usual. The conditions for exposure and development may vary. Immunoprecipitation
1) Wash the cells 3 times with PBS and suspend with 10 volumes of cold Lysis buffer (50 mM Tris-HCl pH 7.2, 250 mM NaCl, 0.1% NP-40, 2 mM EDTA, 10% glycerol) containing appropriate protease inhibitors. Incubate it at 4 oC with rotating for 30 minutes, then sonicate briefly (up to 10 seconds).
2) Centrifuge the tube at 12,000 x g for 10 minutes at 4 oC and transfer the supernatant to another tube.
3) Add primary antibody as suggested in the APPLICATIONS into 200 µL of the supernatant. Mix well and incubate with gentle agitation for 30-120 minutes at 4 oC. Add 20 µL of 50% protein G agarose beads resuspended in the cold Lysis buffer. Mix well and incubate with gentle agitation for 60 minutes at 4 oC.
4) Wash the beads 3-5 times with the cold Lysis buffer (centrifuge the tube at 2,500 x g for 10 seconds).
5) Resuspend the beads in 20 µL of Laemmli’s sample buffer, boil for 3-5 minutes, and centrifuge for 5 minutes. Use 10 µL/lane for the SDS-PAGE analysis. (See SDS-PAGE & Western blotting.) |






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China