PML Protein (PML) Mouse Monoclonal Antibody [Clone ID: 1B9]
Specifications
| Product Data | |
| Clone Name | 1B9 |
| Applications | FC, IF, WB |
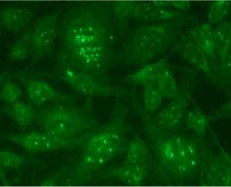
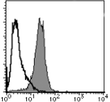
| Recommended Dilution | Western blot: 1 µg/ml (for a chemiluminescence detection system). Immunocytochemistry: 1 µg/ml. Immunostaining features in the APL cell line NB-4 showed the microgranular pattern. Exposure to 0.1 μM of ATRA for 48 hours restored the normal immunostaining pattern. Flow cytometry: 10 µg/ml. For details see protocols below. It is reported that this clone 1B9 can be used in Immunoprecipitation in the reference number 1). |
| Reactivities | Human |
| Host | Mouse |
| Isotype | IgG1 |
| Clonality | Monoclonal |
| Immunogen | Recombinant human PML |
| Specificity | This antibody reacts with PML. |
| Formulation | PBS containing 50% glycerol, pH 7.2. Contains no preservative. State: Azide Free State: Liquid Ig fraction |
| Concentration | lot specific |
| Purification | Protein A agarose |
| Conjugation | Unconjugated |
| Storage | Upon receipt, store undiluted (in aliquots) at -20°C. Avoid repeated freezing and thawing. |
| Stability | Shelf life: One year from despatch. |
| Gene Name | promyelocytic leukemia |
| Database Link | |
| Background | Acute promyelocytic leukemia (APL) is associated with a t(15;17) translocation that creates the promyelocyte-retinoic acid receptor α (PML-RAR α ) fusion protein and successfully differentiated by all- trans -retinoic acid (ATRA). PML-RAR α consists of all amino acid of RAR α except the first 59 amino acids and includes its DNA-binding and ligand-binding domains. PML-RAR α contains the functional domains of PML which includes the DNA binding and dimerization property. Thus, the functions of PML and/or retinoid X receptor are sequestrated by PML-RAR α in a dominant negative manner. In APL cells, the PML-RAR α and PML are immunologically localized as microgranules in the nuclei and cytoplasm, whereas in normal cells, PML is immunologically found as a discrete speckled pattern in nuclei. Th e ATRA treatment of the APL cells triggers a reorganization of PML to generate normal localization. Anti-PML antibody is a strong tool for the detection of the chromosomal translocation t(15;17) on the APL cells and/or determination of the sensitivity of the APL cells to the ATRA differentiation of hematopoietic cells and apoptosis. |
| Synonyms | RING finger protein 71, MYL, TRIM19 |
| Note | This product was originally produced by MBL International. Protocol: Immunocytochemistry SDS-PAGE & Western Blotting Flow cytometric analysis for floating cells We usually use Fisher tubes or equivalents as reaction tubes for all steps described below. |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
Be the first one to submit a review






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China