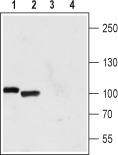
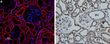
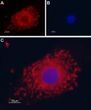
Polycystin 2 (PKD2) Rabbit Polyclonal Antibody
Frequently bought together (1)
beta Actin Mouse Monoclonal Antibody, Clone OTI1, Loading Control
USD 200.00
Other products for "Polycystin 2"
Specifications
| Product Data | |
| Applications | IF, IHC, WB |
| Recommended Dilution | WB: 1:200-1:2000; FC: 1:50-1:600 |
| Reactivities | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Immunogen | Peptide (C)ERWESDDAASQISH, corresponding to amino acid residues 914-927 of human TRPP1. Intracellular, C-terminus. |
| Formulation | Lyophilized. Concentration before lyophilization ~0.8mg/ml (lot dependent, please refer to CoA along with shipment for actual concentration). Buffer before lyophilization: Phosphate buffered saline (PBS), pH 7.4, 1% BSA, 0.05% NaN3. |
| Reconstitution Method | Add 50 ul double distilled water (DDW) to the lyophilized powder. |
| Purification | Affinity purified on immobilized antigen. |
| Conjugation | Unconjugated |
| Storage | Store at -20°C as received. |
| Stability | Stable for 12 months from date of receipt. |
| Gene Name | polycystin 2, transient receptor potential cation channel |
| Database Link | |
| Background | Transient receptor potential (TRP) channels are relatively non-selective ion channels enabling the exchange of cations down their electrochemical gradient. This exchange enables the intracellular rise in Na+ and Ca2+ concentration and ultimately in the cell membrane depolarization, important for action potential propagation and muscle contraction. They are activated by an extremely broad range of stimuli namely, temperature, voltage, pH, endocrine factors as well as signaling molecules. The TRP channel family is composed of 28 members divided in 7 subgroups: TRPV, TRPC, TRPM, TRPA, TRPN, TRPP and TRPML. All members of the TRP family have 6 transmembrane (TM) domains, and a pore domain between the fifth (S5) and sixth (S6) transmembrane domains. In general, TRP channels enable the passage of either Na+ or Ca2+ ions with little or no preference. However, some channels do exhibit some selectivity. Also, TRP channels do not display the positive charges in the S4 voltage-sensing domain like most voltage sensitive channels, although they do display voltage dependency. In addition, TRP channels have in the C-terminal intracellular region to the S6 domain a TRP domain comprising 25 amino acids that is more or less conserved among most TRP channels. Within the TRP domain, there is a TRP box composed of six amino acids, and TRP box 2 – a proline rich domain. The TRP domain seems to be responsible for the binding of PIP2, a phospholipid important for the regulation of channel activity. TRPP1 (polycystin-2, PC2, PKD2) belongs to the TRPP subfamily of TRP channels along with TRPP3 and TRPP5 proteins, and forms non-selective cation channels with different permeability to various divalent cations. The cellular localization of TRPP1 has been and still is the subject of a lasting debate. In many cell-types, TRPP1 is retained in the endoplasmic reticulum (ER) where it most likely functions as Ca2+ release channel, and with the help of cofactors, TRPP2 reaches the plasma membrane and the cilia. TRPP1 expression is widespread and is best characterized for its expression in the kidney where it is developmentally regulated. In the kidney, it associates with PKD1 (TRPP2) an eleven transmembrane-spanning protein (which does not belong to the TRP superfamily) to form functional channels. In addition, TRPP1 is identified as one of the genes responsible for autosomal dominant polycystic kidney disease (ADPKD). |
| Synonyms | APKD2; Pc-2; PC2; PKD4; TRPP2 |
| Reference Data | |
| Protein Families | Druggable Genome, Ion Channels: Transient receptor potential, Transmembrane |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China